An international team of researchers including Professor Ken Duffy from Maynooth University and Dr. Cristina Lo Celso of Imperial College London has made major breakthroughs in the study of acute myeloid leukaemia (AML) which could lead to the improved treatment of sufferers of this aggressive cancer of the blood that affects one in 200 men and one in 250 women in the UK.
One of the reasons this new research is so important is that there is a pressing clinical need for more effective treatments for AML, which has a low cure rate of 5-15% in patients older than 60 and has been treated with essentially the same techniques for the past 30 years.
Their research is the subject of two papers, one of which has been published in the prestigious Cell Stem Cell journal, while the other has been published by high profile journal Nature Communications.
The first study (Nature Communications, 2018) led to the new understanding that leukaemia essentially outcompetes healthy cells for resources, largely oblivious to the fact that healthy cells are being eliminated. The other study (Cell Stem Cell, 2018) subsequently established that a therapy for protecting the bone marrow region that maintains rare blood stem cells, which become heavily occupied by leukaemia, enhanced chemotherapeutic effect and allow blood regeneration.
It had previously been thought that AML actively shut down healthy blood production in the bone marrow. However, the first study (Nature Communications, 2018) established that a simpler high-level mechanism was at play. The researchers found that throughout the disease’s progression, the cancer grew steadily, blithely producing new cells at a constant rate even when it ran out of space in the bone marrow to host them. The remaining healthy cells continued to do their job, but ultimately both healthy and even some cancerous cells were ousted from the bone marrow by the relentless leukaemic growth. While the stem cells that top the hierarchy of healthy blood cell production proved to be the most resistant to this process, they were ultimately eliminated too.
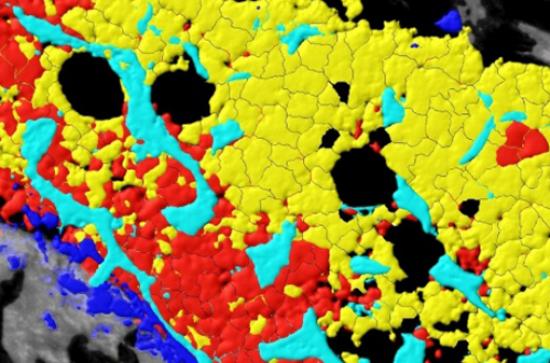
The second study (Cell Stem Cell, 2018) used a distinct set of experimental techniques to directly observe that competition for space, filming the process as it happened. Using intravital microscopy, the team of scientists filmed the progress of AML in the bodies of mice. Using the footage, the scientists observed directly how cancerous cells expanded within the body and gradually killed off healthy blood cells, as well as cells that support blood production and the rare blood vessels associated with blood stem cells. Blood stem cells primarily reside in the bone marrow, and can produce all of the cells found in the blood, including crucial components of the body’s immune system such as cancer-fighting Natural Killer cells. The team found that if they preserved these rare blood vessels through pharmaceutical intervention, they could prevent the disease from wiping out blood stem cells and so improve the efficacy of chemotherapy.
Professor Ken Duffy, Director of the Hamilton Institute, Maynooth University’s interdisciplinary STEM research institute, said the breakthrough could have major implications for future treatments of AML: “Our findings suggest that if we can target treatments that protect the rare blood vessels that are associated with the blood stem cells then we can improve the therapeutic options available to those suffering from AML and, perhaps, other forms of leukaemia.”
“This research is the result of collaboration between researchers and clinicians across the globe from London to Maynooth to Glasgow to Melbourne. I would like to congratulate my computational team at Maynooth, and all of our colleagues involved in this project on what is a fantastic breakthrough,” said Prof Duffy.
Dr Cristina Lo Celso from the Department of Life Sciences at Imperial College London, a lead author on both studies, said of the drug used by the researchers to preserve the key blood vessels: “As the drug in question, Deferoxamine, is approved for human use for a different condition, we already know that it is safe. As it is already in use, it should be possible to progress to clinical trials much quicker than one could with a brand-new drug.”
“Once clinical assessments and human trials start for the use of Deferoxamine in AML treatments, we estimate that determining its viability should take less than five years, in comparison to the 10-15 needed for the development of an entirely new drug.”
This research is supported by Science Foundation Ireland, the University of Porto Graduate Program, the European Haematology Association, the American Society of Hematology, Bloodwise, the Human Frontiers Science Programme, Cancer Research UK, the Biotechnology and Biological Sciences Research Council, the Kay Kendall Leukaemia Fund, the European Research Council, the Wellcome Trust, the Medical Research Council, the National Heart And Lung Institute Foundation, the Royal Society and the Netherlands Organization for Scientific Research.
